How To Test The pH Of Water
Looking to help ensure the quality of your water? Need to test the pH of your water to do so? Then here’s how to test the pH of your water!
You can test the pH of water by using either a pH meter, using universal indicator strips, or litmus paper. A pH meter will need to take several readings to be sure of the results, and universal indicator strips and litmus paper will change color depending on the pH of the water.
The Importance Of Water Quality
Water is an essential part of our lives. It’s not just used for drinking either. It’s also required to perform many tasks, such as maintaining hygiene, cleaning, cooking, and more.
With increased industrial revolution and commercialization, there are a number of polluting sources that don’t just pollute the water but also the air. These pollutants contaminate the water and affect the water quality, which is usually not safe to drink or use for other tasks.
Diseases caused by polluted water, for example, diarrhea, hepatitis A, typhoid, polio, cholera, and dysentery are one of the major concerns in the world, especially in those areas where there aren’t enough facilities for the treatment of water to acceptable quality standards.
Checking Water Quality
Water can be polluted either due to micro-organisms such as E. coli, fecal bacteria, and various chemicals, such as arsenic, fluoride, and nitrates. There are various ways to check the quality of water. However, in general, determining the quality of water requires checking five specific parameters.
These parameters include temperature, dissolved oxygen, turbidity, total dissolved solids, and pH. While all five of these parameters are important, there is one indicator among them that can decide if the water is safe for use or not: pH.
This is because pH is indicative of the chemical condition of the water. Also, pH is an acronym for the potential of hydrogen/ power of hydrogen.
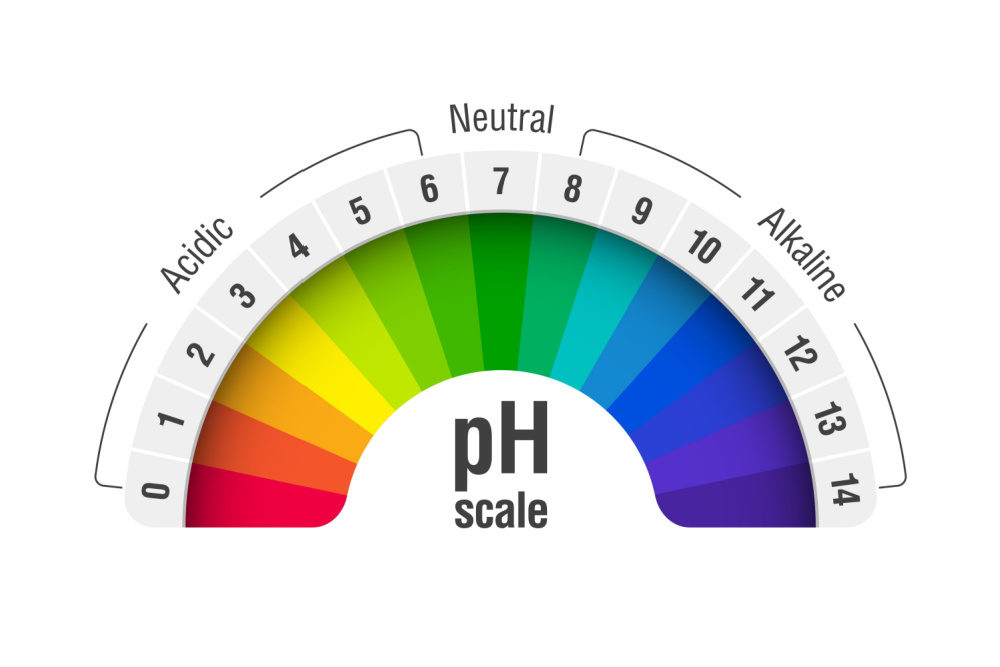
As such, a pH rating ranges from 0 to 14. A value of one to six indicates the water is acidic. However, a value of eight to 14 indicates the water is alkaline. At a pH of seven, the water is neither acidic nor alkaline. Instead, it’s considered neutral. Seven is also the ideal pH value.
The acidity and basicity of an environment matter a lot as they may either promote or slow down a certain activity. For instance, bacteria can live in a certain pH environment only.
It is interesting to note that the different liquids in the human body have different pH values. For instance, the pH of saliva is around 6.4 to 6.7, the pH of stomach liquids is around 1.5 to 3.5, and that of urine normally ranges between 4 to 8 pH. The pH of blood is around 7.35 to 7.45.
As far as the pH of liquids/food we intake, there is no specific defined safe rating of pH that a human body should intake. In our routine lives, we intake a pH range of food and liquid. For instance, the pH of distilled vinegar and lemon ranges from 2 to 2.5, that of milk is 6.7, and 8 for baking soda.
The United States Environmental Protection Agency suggests 6.5 to 9 pH to be safe for drinking but again this is not a hard and fast rule. This is especially true with the growing trend of consuming alkaline water for several supposed health benefits.
How To Test The pH Of Water
There are several ways you can test the pH of the water. Those methods are categorized into two types. The two types are using a pH meter and using pH papers (universal indicator or litmus paper).
Using pH Meters
Many types of pH meters are used on an industrial level, in labs, and for domestic purposes. In labs, rigorous benchtop pH meters are preferred because précised values of pH are required. For simple domestic purposes, simple test kits and easy-to-carry pH meters are available in the market. The accuracy of these meters varies from manufacturer to manufacturer. The instrument becomes costly as the accuracy increases. APULSE, APERA, and Blue Labs are some of the manufacturers that offer a range of portable pH meters in the price range of $75 to $500. Several factors should be considered while purchasing a pH meter.
How To Measure pH Using A pH Meter
Before using a pH meter, it is necessary to compare and set values for the pH meter. This could be done by taking measurements of solutions whose pH is already known. These solutions/powders are provided with pH meters and are called the buffer solution. Now, let’s get into the other steps.
Set The Readings Of The pH Meter
Take a small amount of buffer solution in a cup. The quantity should be enough to dip at least the tip of the pH meter/test kit. So, dip the tip of the pH meter in the buffer solution for 1 -2 minutes or until the reading on the meter is stable. Next, note the reading on the meter. If the reading is not the same as that of the buffer solution, adjust the reading of the meter as per instructions provided by the pH meter instruction manual.
Test The pH Meter With A Neutral Buffer Solution
A pH meter usually comes with three different buffer solutions with different pHs: one acidic, one basic, and one neutral. After setting the readings of the pH meter, put the neutral buffer solution in a cup. Again, the quantity should be enough to dip the tip of the test kit. Dip the pH meter tip in the pH 7 buffer solution. Allow it to remain in the solution for a few minutes until the reading is stable on the meter. The reading should be seven and if it is not, adjust the readings as per instructions provided in the instructions manual.
Rinse the tip of pH water, preferably with distilled water. Now the pH meter is ready to be used to measure the pH of water.
Test The pH Of The Water
Take the water sample with the pH to be checked in a cup. The quantity should be enough to dip the tip of the pH meter or else it will give an error. Dip the tip of the pH meter into the water and allow it to sit for a few minutes until the reading is stable on the meter. Note down the reading from the meter.
Repeat this twice for accurate results. Sum up all these readings and divide them by the number of times the readings are taken. Three, for example. This will give an average of the readings.
The average of readings is the pH of the water.
Checking The pH Of Water Using pH Strips
The idea behind using pH Strips is that the strips change color when introduced to different pHs. Each color indicates a certain value of pH. There are two types of strips used to check the pH.
Universal Indicator pH Strips
A pH universal indicator strip is made from a mixture of several chemicals that include but are not limited to sodium bisulfate, methyl red, sodium hydroxide, water, bromothymol blue, phenolphthalein, and 1- propanol that react and change color in a different pH. The strip changes color as per the different pH of the solution. The color is compared with a scale that shows what color means what pH.
To check the pH of water using pH strips:
- Take the sample to test in a cup, the quantity should be enough to dip at least half of the strip.
- Keep the strip in the sample for a few seconds until it changes color.
- Compare the strip color with the chart provided with the pH strips.
- The chart will indicate a specific pH value against the color. That color rating is the pH of water.
Checking pH Using Litmus Paper
A litmus paper is wooden cellulose paper infused with litmus – a mixture of weak acids and basics formed from lichens. There are two types available. One is blue and the other one is red. The blue litmus changes color to red if the water is acidic and the red litmus strip changes to blue if the water is alkaline. If the water’s pH is neutral (pH 7), the litmus paper will not change color.
When Should You Test The pH Of Water?
There are some visible factors when one should consider checking the pH of water. Rusty faucets, green and blue tans on copper piping, and leakage are due to the acidic nature of the water. The acidic nature of water is due to the addition of metals in the water.
Conclusion: How To Test The pH Of Water
So, there you have it. That’s the way to test the pH of water. This is an important part of water quality, and we hope this will help you to improve and maintain yours.
So, have you ever tried to test the pH of water before? Are you planning on it? Let us know in the comments below!








